Background: While the standard regimen for AML has been intense chemotherapy followed by stem-cell transplantation (SCT), addition of immunotherapeutics such as gemtuzumab ozogamicin (GO) to this has shown to improve outcomes in multiple clinical trials. Since these trials, GO has been added to the National Comprehensive Cancer Network (NCCN) practice guidelines for use in AML therapy (NCCN., 2018). GO is a humanized monoclonal antibody conjugated to calicheamicin and targets the cell surface antigen CD33, observed on most AML blasts. Upon binding to GO, CD33 is internalized and the subsequent release of calicheamicin mediates cytotoxicity. CD33 contains two extracellular domains: IgV and IgC, the IgV domain is coded by CD33 exon 2 and is the binding site for GO. A splicing SNP, rs12459419 (C>T; A14V) in exon 2 results in a shorter isoform (CD33-D2) and has been previously associated with CD33 abundance on cell surface as well as clinical benefit from GO addition to standard chemotherapy. Patients with CC genotype (~50% of the cohort) expressed full length CD33 (CD33-FL) and benefited significantly from GO. However, heterozygous CT and homozygous TT genotype groups did not derive the same benefit. These results were perplexing as CT genotype group had intermediate levels of CD33-FL and CD33-D2 isoforms (Lamba et al., 2017). It is hypothesized that lack of IgV domain in CD33-D2 and putative CD33-FL/CD33-D2 dimerization prevents GO binding and subsequent internalization in rs12459419 CT/TT genotype cells, however this has never been tested. We have generated novel antibodies directed against the IgC domain that recognizes CD33-D2 in AML cell lines as well as primary AML cells (Gbadamosi et al., 2021) thus facilitating testing the above indicated hypothesis.
Objective: In this study, we first tested the ability of antibody-bound CD33-D2 to internalize, followed by impact of the internalization status of CD33-FL and CD33-D2 in cells co-expressing both isoforms along with the concomitant impact on GO induced cytotoxicity.
Methods: Using pMXs retroviral particles encoding CD33-FL or CD33-D2, we engineered CRISPR/Cas9 mediated CD33 KO in AML cell lines to overexpress either one or both CD33 isoforms to mimic cells expression varying proportions of CD33-FL/CD33-D2. Internalization of either CD33-FL or CD33-D2 over a duration of 4 hours in the presence of antibodies recognizing either of the two isoforms was performed. Cells were labeled with unconjugated mouse anti-CD33-FL P67.6 or anti-CD33-D2 HL2541 antibody on ice and incubated at 37°C for 0-4 hours, followed by staining with mouse anti-mouse IgG antibody conjugated with biotin. Surface CD33-FL or CD33-D2 was then detected using streptavidin-FITC conjugate. Percentage antibody-bound CD33-FL or CD33-D2 internalization was expressed as change in FITC MFI between each time point incubated and cells kept at 0°C post CD33 antibody labeling.
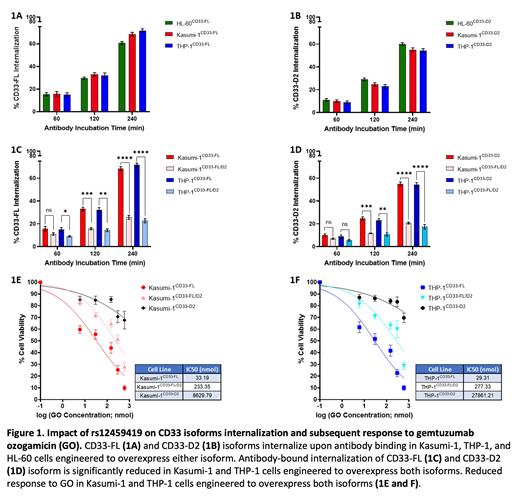
Results: While P67.6-bound-CD33-FL internalized as expected in AML cell lines expressing CD33-FL only (Fig. 1A), we show for the first time that HL2541-bound-CD33-D2 also internalizes upon antibody binding in cell lines expressing CD33-D2 isoform only (Fig. 1B). In AML cell lines co-expressing both CD33-FL and CD33-D2 isoforms, a >2.5-3 fold decrease in this antibody-bound internalization was observed for each isoform (Figs. 1C and D). In response to GO exposure for 48 hours, cells co-expressing both CD33-FL and CD33-D2 isoforms had 7-9.5-fold higher IC50 values (233 nmol and 277 nmol) compared to cells expressing only CD33-FL (33.19 nmol and 29 nmol) (Figs. 1E and F). As expected, cells expressing CD33-D2 only did respond to GO.
Discussion: Our finding that antibody-bound CD33-D2 internalizes like CD33-FL adds a novel piece of evidence in understanding the biology of this variant isoform and opens opportunities to develop antibody drug conjugate (ADC) such as GO using CD33-D2 antibody. This new CD33-D2 targeting ADC will provide a treatment option in patients with TT genotypes. Our results also provide a mechanistic insight on the previous clinical observation showing lack of benefit from GO in patients heterozygous for the splicing SNP. Our ongoing studies are focused on validating this result using live cell imaging and efforts towards design of new ADC or T cell engagers using CD33-D2 antibody.
The study was supported by Leukemia Lymphoma Society, UFHealth Cancer Center, and the Frank A. Duckworth Endowment.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal